For Consultation:
Artificial Cervical Disc - Cervical & Lumbar
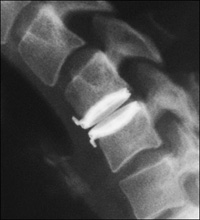
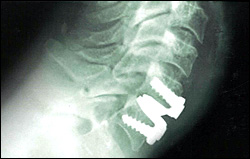
Artificial Cervical Disc:
FDA-Approved Artificial Cervical Disc-
The PRESTIGE® cervical disc, a product of Medtronic, is the first and only artificial disc to be approved by the Food and Drug Administration (FDA) for use in the cervical spine, as of July 2007. The PRESTIGE disc is indicated for spinal arthroplasty in skeletally mature patients with degenerative disc disease at one level from C3 to C7, for intractable radiculopathy and/or myelopathy. This is a stainless-steel device with a ball-in-trough design, held in place with bone screws.
The BRYAN® cervical disc, also
The goals of artificial cervical disc surgery are to: 1) Remove the diseased disc; 2) Restore normal disc height; 4) Decrease discogenic neck pain and associated arm pain/weakness; 4) Preserve motion in the affected vertebral segment; and 5) Improve patient function. In comparison to spinal fusion surgery, potential benefits of artificial disc technology may include more spine mobility after surgery and less stress on adjacent discs. While cervical artificial discs have been shown to preserve motion at the operated segment in most patients, their effectiveness in reducing the rate symptomatic adjacent disc problems has not been established.
To be considered a candidate for artificial cervical disc, you must meet the following specific criteria:
• Disc degeneration in only one disc in the cervical spine.
• A minimum of six months of conservative treatment, such as physical therapy, pain medication, or neck bracing, without showing improvement.
• Overall good health with no signs of infection, osteoporosis, arthritis, or osteomalacia
• No known allergies to stainless steel.
If you have degeneration affecting more than one disc, segmental instability, any type of metabolic or hereditary/acquired bone disease, you are not a candidate for this surgery. This surgery is not recommended in patients who have undergone prior spinal fusion/surgical procedures at the same or adjacent cervical levels.
During surgery, the patient is under general anesthesia and a small incision is made in the front of the neck. Through this opening, the affected disc is removed and replaced. The average hospital stay postoperatively is about 1-2 days. Although a similar artificial cervical disc has been used in Europe since 2004, there is very little information available on the number of surgeries performed to date.
FDA-Approved Artificial Lumbar Discs:
The CHARITÉTM artificial disc, a product of DePuy Spine, a Johnson & Johnson company, was the first approved by the Food and Drug Administration (FDA) in October 2004. The CHARITÉ disc is indicated for spinal arthroplasty in skeletally mature patients with degenerative disc disease at one level from L4 to S1. The mobile-core design consists of a sliding plastic core between two chrome plates.
The ProDiscTM-L Total Disc Replacement, a product of Synthes Spine, was approved by the FDA in August 2006. The ProDisc-L is indicated for spinal arthroplasty in skeletally mature patients with degenerative disc disease at one level from L3 to S1. The fixed-core ball and socket design consists of three implant components – two chrome endplates and a plastic inlay.
Artificial Disc Surgery-
Artificial lumbar disc surgery is an alternative to spinal fusion surgery, a common operation performed on about 200,000 people a year with degenerative disc disease in the lumbar spine (lower back). Spinal fusion surgery creates a solid union between two or more vertebrae to help strengthen the spine and alleviate chronic back pain. There are many types of spinal fusion surgery, as well as varied instrumentation used to secure the fusion. The spine is accessed either through the back or abdomen, depending on the type of spinal fusion procedure.
The goals of artificial lumbar disc surgery are to: 1) Remove the diseased disc; 2) Restore normal disc height; 4) Decrease discogenic back pain; 4) Preserve motion in the affected vertebral segment; and 5) Improve patient function. In comparison to spinal fusion surgery, potential benefits of artificial disc technology may include quicker recovery time, more spine mobility after surgery, less stress on adjacent discs, and no need to harvest and use a bone graft.
To be considered a candidate for artificial lumbar disc surgery, you must meet the following specific criteria:
• Disc degeneration in only one disc in the lumbar spine, between L4 to S1 or L3 to S1.
• A minimum of six months of conservative treatment, such as physical therapy, pain medication, or back bracing, without showing improvement.
• Overall good health with no signs of infection, osteoporosis or arthritis.
• No known allergies to cobalt, chromium, molybdenum, polyethylene or titanium
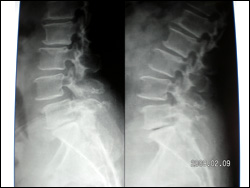
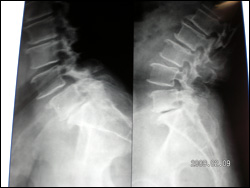
Dynamic Fixations of The Lumbar Spine (DIAM)
This elderly lady was unable to stand, or walk due to incapacitating back pain. She had degenerative disorder of the lumbar discs leading to retrolisthesis and diminished disc space along with interspinous laxity and instability. She underwent an interspinous dynamic stabilization with DIAM at L4 and L5 interspinous area. This is a synthetic flexible implant (Medtronic Inc.) that allows a dynamic stabilization of the interspinous area in such cases and a symptom free life. Interbody fusion and pedicular fixation need not be done in such cases as these are essentially non dynamic fixations.
Contact Details
Mobile: +91 98308 34566
Email:
pbishnu04@yahoo.co.in
pbishnu08@gmail.com
Website:
www.neurosurgeryindia.co.in